classification of periodic table
Here we will learn about periodic table in simple language .We all are familiar with the different type of elements which has different property .Can you ever thought why we study the classification of these elements ? let's find its answer ...Need of Classification : In order to systematic study of elements we need to classify elements on the basis of their properties.
"hence periodic table may be defined as the arrangement of element on the basis of their properties."
Antoine lavoisier was the first scientist who classified elements into metal and non-metal but later on this classification was failed in case of metalloids.
Properties of metal
1. Metals are hard and having high melting and boiling point .
2. Metals have metallic lustre.
3. Metals conduct heat and electricity.
4. Metals are malleable and ductile.
5. Metals produces sound when strike another metal.
Well, now we are going to move to the definition of periodic table
Periodic Table : It is a synthetic arrangement of element in the tabular form to study the physical and chemical property.
1.Lavoisier Classification
He classify the element into metal and non-metal form. This classification was based on certain distinctive physical properties such as hardness, ductile, conductance malleability and luster. On the basis of these properties, sodium and lead were classed together as belonging to the group of metals.Lavoisier classification was not applicable on general property.So it is not much important for us.
Now we are moving to another classification given by Dobereiner.
2.Dobereiner triad (1815-1829)
According to dobereiner tried the set of three elements put together in the same group whose physical and chemical property are same.
for example : Cl Br I
35.5 80 127
It was a good approach in classification but not the successful step.
Now we're moving to another classification.
3.Newland's Law of Octet
Newland classify the periodic table on the basis of musical note in which every eight element will repeat its physical and chemical property.
Sa Re Ga Ma Pa Dha Ni
Li Be B C N O F
Na Mg Al Si P S Cl
K Ca ------->
But it is not valid for next more so we just know it for general knowledge.
4.Lother Mayer
He classify the periodic table on the basis of atomic volume i.e V= m/D (here m is mass of the element and D is the density of that elements).
This classification was applicable for physical property of the element.
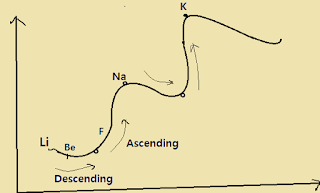
*The pick of the curve is occupied by alkali metal ( Li,Na,K,Rb,Sc).
The minima of the curve is occupied by transition metal ( Mn, Cr, Fe, Co, Ni ) .
The descending line represent the alkaline earth metal ( Be, Mg, Ca ) whereas ascending line represent halogen ( F, Cl, Br, I ).
Here important note is this curve is not useful for chemical property.
5.Mendeleev's Periodic Table (1869)
Mendeleev classified the elements on the basis of atomic mass of the elements and it was a good approach that gives idea to design the modern periodic table.
Periodic law : "According to Mendeleev's the physical and chemical properties of the elements are the periodic function of atomic mass."
Mendeleev defined the vertical column as the group in which element will have the same physical and chemical property.
I H
|
|||||||
Li
|
Be
|
||||||
Na
|
Mg
|
||||||
K Cu
|
Ca Zn
|
||||||
Rb Ag
|
Sr Cd
|
||||||
Cs Au
|
Ba Hg
|
Tl La*
|
Mendeleev's periodic table was classified into 8th vertical column on the basis of valency of the elements.
Drawback of Mendeleev's periodic table
1.Position of hydrogen : In the Mendeleev's periodic table the position of the hydrogen is controversial .Some of the properties of hydrogen are similar as 1st group as well as some of the properties are similar to 7th group of the table.
2.Anomalous Pair : In Mendeleev's periodic table ,some of the element having lower atomic weight is placed after the element having higher atomic weight.
for example --- Te I
127.6 127
3.Some of the transition metal are placed together with different element having different physical and chemical property.
Ex. - Cu, Ag, Au are placed together with alkali metal.
4.Their is no explanation for the position of isotope in the periodic table.
5.Some of the element which have the same property are separated in the Mendeleev's periodic table .
Ex. -- Ba and Pb
6.No justification about the position of lanthanide and actinide.
Almost Mendeleev gave the basic idea to form a periodic table and so it is important to understand the Mendeleev's classification before go further.
Almost Mendeleev gave the basic idea to form a periodic table and so it is important to understand the Mendeleev's classification before go further.
6.Modified Periodic Table of Mendeleev or Modern Periodic Table
Go further to continue this topic by following link:
https://shrischool.blogspot.com/2020/05/modern-periodic-table.html

No comments:
Post a Comment
If you have any doubt don't hesitate to write in comment section.I will always answer your doubt.